Please click on a question below to see the answer.
All questions about Flashkit
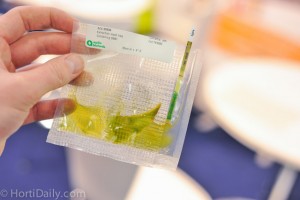
Why does the ImmunoStrip have no control line or a weak control line?
This means the test result is invalid. An invalid test result is most generally caused by oversubmersion of the test strip in the sample. When running an ImmunoStrip test, make sure the test strip is only submerged about 1/4″ into the sample. The furthest a test strip should be inserted into a sample is to the white line on the green label located below the sample arrows. Other causes, although very rare, could be the test is not compatible with the sample type you are testing, the test was not stored properly, or the test is past its expiration date. For more information please contact us.
Why do my test result appear to be weak? Is there a problem with my test?
Is your control line darker than the test line and clearly visible? If not the test may be invalid. Several factors can cause an invalid test, including improper storage and submerging the ImmunoStrip too far in the sample extract.If the control line is valid, then you may be seeing a light positive that is caused by a low titer sample. If the pathogen or analyte titer is too low, or approaching the tests LOD (Limit of Detection), weak results are expected.One remedial approach would be to retest a fresh sample taken from symptomatic leaves. Although many of our ImmunoStrip tests can reliably detect asymptomatic samples, the sample may be newly infected and the pathogen concentration may be too low for detection.
I left my ImmunoStrip container out of the refrigerator overnight. Are my tests still usable?
Providing the test container was sealed and desiccated, the tests should still work fine. All of our ImmunoStrip tests undergo rigorous stability studies to ensure they can withstand periods of time outside of our recommended storage conditions, such as shipment.
However, if your container was left open there is a chance they have absorbed moisture and may no longer work according to our quality assurance standards. Please be sure to always keep the strip container closed when not in use.
How reliable are my test results?
Agdia puts a great deal of effort into developing all of our ImmunoStrip tests. We strive to ensure that our ImmunoStrip tests are as sensitive and accurate as their ELISA counterparts, where available. In fact, many customers are not aware that they can achieve the same level of reliability and confidence, while saving time and money, using ImmunoStrip tests compared to ELISA methods.
During the development process we look at as many analyte and host variations as possible to ensure the highest level of accuracy. If we are aware of any non-specific reactions to a sample matrix or other analyte, it will be posted in the test user guide. All of our ImmunoStrip tests are guaranteed to meet QA specifications for one year from the date of purchase.
There is a green line where the test line should be. What causes this and how should I interpret the result?
Most often, « green lines » are caused because the sample extract is too concentrated. To fix this problem, you can try further diluting your existing extract with buffer, or prepare a new extract using less plant tissue.
If you are unable to retest the extract in one of the ways described above, the result can be interpreted as negative providing it has no red or purple hue. However, we strongly encourage a retest when this occurs because the green line can potentially mask low level positive results.
At test completion, I interpreted my result as negative. Now, the test has dried and I can see a line. What does this mean?
This can mean a number of things. However, in most cases this anomaly is caused by what we call « secondary flow » and commonly occurs when the sample pad is not cut off at test completion. If the pad is not cut off, liquid in the sample pad will continue to flow up the strip as it is drying which may cause a light false positive to appear. In this case, we recommend retesting the sample and cutting off the sample pad after you have interpreted your result according to test instructions.
Results interpreted after the assay time has expired are not as reliable as when completed per the test instructions.
All questions about ELISA

Why isn’t my ELISA producing any color?
You may not have added the enzyme conjugate.
You may have used the capture antibody instead of the enzyme conjugate.
The positive control may be inactive.
You may not have added the PNP substrate tablet to the PNP substrate buffer.
Can I coat plates and then store them?
All Agdia plates can be stored for longer periods of time if postcoated with the appropriate postcoat buffer. Please contact Agdia directly for more information.
Why did color appear in all my testwells?
You may not have washed the plate properly.
The substrate solution may have been contaminated. PNP tablets can be contaminated by touching them.
The substrate solution or plate may have been exposed to bright light.
You may have added positive samples to all wells.
You may have used an old buffer that has become contaminated.
(Reagent sets only) You may have coated the plate with enzyme conjugate, or with coating antibody contaminated with enzyme conjugate. You must use only new, sterile pipette tips.
Why did color develop in all wells along the edge of the plate?
You may not have washed the edge wells properly.
Lower quality plates may cause an edge effect. This is not a problem with high-quality new plates such as those supplied by Agdia.
Why did unexpected color develop in wells near positive samples or positive controls?
You may have contaminated surrounding testwells during the wash step. Please see our videos on ELISA use in the
“How to” video section.
Why did I get what appears to be false positive results with my samples?
The plant sample is really positive.
Some carryover may have occurred on your grinding equipment.
Why are my positive results weak?
Your kit components may have expired.
The pathogen titer or analyte titer may be low.
You may have used an inappropriate sample extraction buffer.
(Alkaline phosphatase tests) You may have prepared the PNP buffer using PBST wash buffer instead of distilled water. Phosphate in PBST can reduce the amount of color produced.
You may have over diluted the enzyme conjugate or substrate.
Why does one of my test components have an odor of decay?
The test component may be contaminated and should not be used.
How long does it take to perform an ELISA test?
Agdia leads the way with ELISA protocols that are flexible and reliable. Our standard PathoScreen can be completed within 5 hours (sample 2hrs or overnight, conjugate 2 hours, substrate 1 hour). Reagents can be completed between 6 and 8 hours (coat 4 hours or overnight, sample 2 hrs or overnight.
What are the ideal temperature ranges for storing and performing the test?
Agdia products have been developed to be highly sensitive and specific while also being robust enough to withstand temperature fluctuations and unrefrigerated storage during shipping. The storage and incubation temperatures listed on our products and user guides reflect the average conditions observed in our laboratories. To ensure that your test is performing optimally, we recommend storing the refrigerated components at 2 – 8 °C. When performing the test or storing components at room temperature, we recommend a range of 21 +/- 3 °C. In many situations, temperature fluctuations beyond those listed above may not significantly affect the outcome of a qualitative test, however, Agdia can only guarantee test performance within the recommended temperature ranges. We understand that temperatures at your location may be different from those in our test validation laboratory, so if you have concerns about your local conditions, please feel free to contact info@agdia-emea.com.
All questions about AmplifyRP®

What advantages does AmplifyRP® offer compared to conventional or real-time PCR?
Several. Although PCR is a highly sensitive and specific type of DNA testing, it does require some skill and is typically restricted to laboratory environments. In most PCR assays it is first required that suspect samples undergo a DNA purification or “clean-up” step. This step requires the use of expensive purification kits and sometimes toxic chemicals. PCR also requires an important step called thermal cycling where reactions are repeatedly subjected to temperature fluctuations that facilitate the actual PCR process. To summarize, PCR requires expensive equipment, skilled technicians, and several hours to complete.
AmplifyRP® provides a DNA testing platform that rivals PCR in terms of sensitivity and specificity, but it eliminates the need for expensive equipment and can be completed by users of all skill levels…. all in about 30 minutes
• The total cost for equipment is as low as $300 depending on the format
• Crude extracts may be used for analysis
• Total assay time is between 25 and 40 minutes, including sample preparation
• Format is user-friendly and can be field portable
Do I need special training to use AmplifyRP®?
No. AmplifyRP® was designed with multiple types of end users in mind. All the reagents and disposable equipment necessary to run an AmplifyRP® test comes included in a kit. Any equipment required can be purchased upfront in a starter pack.
AmplifyRP® kits were also designed to limit the number of steps in the protocol. All the amplification reagents are lyophilized in one-time use tubes. The only thing the end user needs to do is rehydrate the reagents and insert their sample. Once that is complete, amplification and detection can be completed in 15 to 30 minutes depending on the format.
What part of my ground sample should I use to test?
With your sterile loop, please take your extract from the fully mixed sample solution. Do not add large pieces of tissue to the reaction.
What happens if I add more than 1 µl per reaction?
The amount of sample extract that you add to a reaction is approximately 1µl. Small changes to volume added (1µl or less) do not affect test performance.
Can I use an extraction buffer other than the recommended buffer in my test?
Only the extraction buffers supplied with the kit are validated with the test. Grinding tissue in any other buffer may lead to false negative results.
What happens if I incubate the reaction at a high temperature?
If you incubate your reaction at a temperature significantly higher than 39°C (42°C or greater), the enzymes in the pellet will become inactive increasing the chance of a false negative result.
What happens if I incubate the reaction at a low temperature?
If you incubate your reaction at a temperature lower than 39°C, the reaction will occur more slowly. Since a lower temperature incubation requires a longer incubation time, adding the reaction to the detection chamber too soon can lead to a false negative result.
What happens if the heat supply has been interrupted?
If your heat supply has been interrupted during the reaction or if you’ve mixed your extract with the reaction pellet without immediately heating the reaction, the test may still be valid. After you have noticed that the heat supply has been interrupted or the heat block was not turned on, place your reaction back in your heat source for an additional 15 minutes and proceed with the instructions as recommended.
Do you have a protocol for insect or other tissues?
If an extraction protocol for your tissue type is not specifically described in your user guide, please contact us by e-mail info@agdia-emea.com or phone +33 (0) 1 60 78 81 64. for more information.
Can I retrieve the strip from the detection chamber for my own records?
Once you have closed the detection chamber, do not attempt to re-open the device. Opening or mishandling the chamber from a completed reaction could lead to contamination of the work area and false positive results. If you would like to keep a record of the results, Agdia-Emea recommends keeping a photographic record.
What should I do if my reaction tube lid pops open before I secure it in the chamber?
If a tube from a completed reaction opens before it is secured in the detection chamber, please discard the test and clean the area. If you suspect you have exposed your work area to the contents of a completed reaction, wash your hands and wipe nearby surfaces with a bleach solution before performing your next test.
How do I dispose of the completed test reaction?
The detection chamber and associated materials can be disposed as general waste. Do not disassemble the detection chamber.
Does this test include an internal reporter (housekeeping) control like some PCR reactions?
The control line in an Acceler8 assay is a measure of the performance of the lateral flow strip, not the nucleic acid extraction. Internal reporters or housekeeping primers are used in PCR reactions to detect the presence of host (plant) nucleic acid following nucleic acid purification. AmplifyRP® technology utilizes crude plant extracts and does not require any purification technique that could lead to the loss of host DNA or RNA, so internal reporters are not used.
What should I do if the product appears damaged?
Contact Agdia Biofords immediately if you notice any defect to the packaging or kit components or missing item.
Who can I contact if I have questions about the test performance?
Please contact contact us by email info@agdia-emea.com or phone +33 (0) 1 60 78 81 64.






